Technology Behind Recombigain
The need for a safe, affordable and effective periodontal regeneration product, has been the driving force behind the development of Recombigain.
- Untreated periodontal disease leads to tooth loss through destruction of the attachment apparatus and tooth-supporting structures.
- The goals of periodontal therapy include not only the arrest of periodontal disease progression, but also the regeneration of structures lost.
- Conventional surgical approaches (e.g., flap debridement) continue to offer time-tested and reliable methods to access root surfaces, reduce periodontal pockets, and attain improved periodontal architecture. However, these techniques offer only limited potential towards recovering tissues destroyed during earlier disease phases
- The prevalence of dental diseases is increasing due to the growth in risk factors such as aging, diabetes, poor oral hygiene, stress, and tobacco & alcohol use.
- Severe periodontal disease is the sixth most common disease worldwide as approximately 10% of the global population were affected by some type of periodontal disease
A Gel based thermo-sensitive composition which contains rAmelX, was developed.
rAmelX is a highly purified human recombinant amelogenin produced by genetic recombination technology using E. coli expression system.
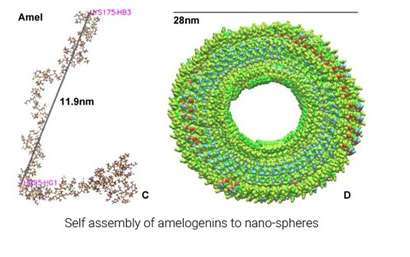
The protein wasincorporated into a patented single use formulation injection to induce healing and regeneration.
The regenerated tissues include tooth-supporting tissues: alveolar bone, periodontal ligament (PDL) and connective tissues and cementum.
Amlogenin is a protein which allows tissue regeneration when it is introduced to a damaged area. It acts both as an attachment factor and as an attractant for mesenchymal stem cells.
Following recruitment of the cells, a regenerative response is induced. The MSCs later proliferate and differentiate into the targeted cell types and tissue layers which fully rebuild the injured area without creating scar tissue.
Recombigain is an adjunct to periodontal flap surgery as a sterile topical application onto exposed root surfaces to induce the regeneration of periodontal tissues: cementum, periodontal ligament, alveolar bone and gingival tissues.
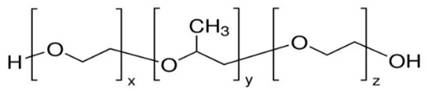
The thermo-sensitive gelling agentchosen: poloxamer 407, triblock copolymer consisting of a central hydrophobic block of polypropylene glycol flanked by two hydrophilic blocks of polyethylene glycol. It has unique phase changing properties when formulated at specific concentrations: a low viscosity liquid and easy to administer at refrigerated or RT conditions that transforms into a solid gel at body temperature.
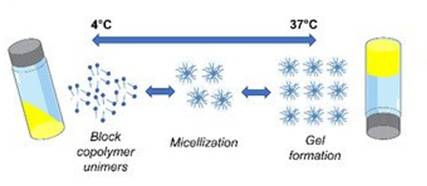
After 6 years in R&D, rigorous tests and stability studies, the optimal formulation has been selected and tested in preclinical trials at first and later in human clinical trials with safety and efficacy goals achieved, granting a medical device registration for clinical use.
Preclinical results have demonstrated the safety and efficacy of Recombigain on recession defect healing (maxilla) and periodontal regeneration (mandible) was assessed twelve weeks after one single treatment.
A histological processing was performed followed by histological and histo-morphometric analysis. For comparative evaluation, defects in both the mandible and maxilla were treated with Recombigain (test) or with Placebo composition (carrier only, control). No adverse effects were observed, and all defects healed.
Recombigain bone gain group was better than placebo, both in the mandible and maxilla.
Recombigain cementum group gain was better than placebo, both in the mandible and maxilla
Recombigain has the following advantages:
- Novel technology – The protein is manufactured in a recombinant production process – no animal source ingredients
- Purity- A single active ingredient carefully crafted into a patented formulation
- Efficacy – A potent rAmelX enamel matrix amelogenin protein
- Non leak formula-A thermodynamic formulation: liquid in the refrigerator and a thick viscous mucoadhesive gel in body temperature. Transforms in seconds
- Reproducibility –A batch consistent production process with exactly 0.5mg/g rAmelX per syringe
- Cost-effectiveness– One syringe per package and a one step application
(no need for a pre-application gel)
